1、引言
3D打印又稱增材制造(Additive Manufacturing, AM),其思想在19 世紀(jì)首次被提出,但真正有一個(gè)質(zhì)的飛越是在20 世紀(jì)80 年代。2010 年,報(bào)道稱美國(guó)公司的3D生物打印機(jī),已經(jīng)能實(shí)現(xiàn)20μm的打印精度,能夠打印出多種人體組織和器官[1]。發(fā)展至今,3D打印已經(jīng)能制造一定精度的產(chǎn)品,并逐漸被廣泛使用[2]。由于其強(qiáng)大和高效的工業(yè)制造能力,3D打印成為了最具前途和革命性的技術(shù)之一[3]。金屬3D打印出現(xiàn),解決了很多醫(yī)用領(lǐng)域的難題,備受追捧[4]。在醫(yī)療領(lǐng)域,該技術(shù)在人工骨的制備上也發(fā)揮了極大的價(jià)值。人工骨材料被植入人體,用于修復(fù)骨缺損,直接與人體組織直接接觸,替代缺損骨骼實(shí)現(xiàn)正常骨骼的功能,所以其必須符合醫(yī)用材料的使用要求[5]。TC4鈦合金,也稱5級(jí)鈦(Ti-6Al-4V),是鈦合金的一種,它因具有優(yōu)秀的力學(xué)強(qiáng)度、耐腐蝕性能、生物相容性等,被廣泛應(yīng)用于醫(yī)療領(lǐng)域,常被作為人工骨材料,植入人體治療骨缺損[6] [7]。遺憾的是,致密的TC4鈦合金彈性模量仍然過高,作為骨科植入物時(shí),高于人骨的彈性模量會(huì)帶來“應(yīng)力屏蔽”效應(yīng),造成植入物周圍骨組織流失,最終導(dǎo)致植入物失效[8] [9]。TC4鈦合金的另一個(gè)關(guān)鍵的缺點(diǎn)是表面缺乏生物活性,作為人工骨植入骨組織,與自然骨的骨結(jié)合不夠穩(wěn)固,容易在承受載荷的時(shí)候造成松動(dòng)[10]。松動(dòng)對(duì)人工骨來說是嚴(yán)重的,它會(huì)導(dǎo)致種植體的失敗。致密鈦合金的缺點(diǎn)導(dǎo)致這種優(yōu)良的金屬材料被限制使用。然而目前沒有發(fā)現(xiàn)更完美的替代材料,于是研究人員們一直在致力于更正TC4鈦合金的缺點(diǎn),并繼續(xù)使用它。為了降低鈦合金產(chǎn)品的彈性模量,并一定程度上改善其生物活性,人們提出了多孔結(jié)構(gòu)的鈦合金材料,該方法被很多學(xué)者證實(shí)確實(shí)行之有效[11] [12]。多孔結(jié)構(gòu)的設(shè)計(jì)補(bǔ)償了TC4鈦合金一部分缺點(diǎn)。傳統(tǒng)的制造工藝,制造孔隙率可控的多孔結(jié)構(gòu)仍然困難,3D打印的出現(xiàn)使人們看到新的希望,但目前該技術(shù)仍面臨著產(chǎn)品質(zhì)量不夠理想、產(chǎn)品性能令人擔(dān)憂等有待解決的問題和挑戰(zhàn)。本文主要從3D打印的TC4鈦合金的優(yōu)勢(shì)、產(chǎn)品質(zhì)量、機(jī)械性能、生物相容性、生物安全性、耐腐蝕性能和表面改性進(jìn)行綜述,并引用多例臨床使用的3D打印制造的TC4鈦合金人工骨的治療效果進(jìn)行舉證,以說明3D打印TC4鈦合金人工骨的可行性。
2、3D打印醫(yī)用鈦合金的優(yōu)勢(shì)
在 3D打印技術(shù)出現(xiàn)以前,傳統(tǒng)的醫(yī)用領(lǐng)域的TC4鈦合金產(chǎn)品的制造方式大多是通過減材制造的方式生產(chǎn)。對(duì)于批量生產(chǎn)的TC4鈦合金件,減材制造更加快速,且成本低。但是對(duì)于人工骨,不需要批量制造,因?yàn)樗男枰莻€(gè)性定制。對(duì)于制造復(fù)雜、不規(guī)則的結(jié)構(gòu),傳統(tǒng)的加工方式受到很大的限制,而且對(duì)非標(biāo)、非批量需求的產(chǎn)品,傳統(tǒng)的制造技術(shù)制造的成本高。在醫(yī)用領(lǐng)域,制造人工骨結(jié)構(gòu),減材制造的方式不具備成本優(yōu)勢(shì),3D打印技術(shù)則可在較大程度上解決相關(guān)問題。將鈦合金種植體制造成多孔結(jié)構(gòu)能降低種植體的彈性模量,進(jìn)而降低由于彈性模量不匹配而引起的“應(yīng)力屏蔽”效應(yīng)[13] [14]。“應(yīng)力屏蔽”效應(yīng)是一種缺點(diǎn),會(huì)促進(jìn)的植入物周圍的骨流失,骨流失會(huì)導(dǎo)致種植體的失敗[15] [16] [17] [18]。李、鄭、孫等學(xué)者[19] [20] [21]認(rèn)為,表面具有多孔結(jié)構(gòu)的TC4鈦合金,還能一定程度上改善表面的生物活性,更適合成骨細(xì)胞的增殖分化,骨整合能力更佳。而比起減材制造,增材制造制備孔隙可控的多孔鈦合金件更加簡(jiǎn)便[22],簡(jiǎn)便的工序必將帶來成本優(yōu)勢(shì)。
3、3D打印醫(yī)用鈦合金人工骨的性能
3.1. 產(chǎn)品質(zhì)量
先進(jìn)的加工工藝,首先需要解決的是產(chǎn)品的質(zhì)量問題。在醫(yī)用領(lǐng)域,3D打印的TC4鈦合金人工骨,根據(jù)設(shè)計(jì)的模型進(jìn)行制造,產(chǎn)品的各個(gè)尺寸的誤差必須在可接受的范圍內(nèi)。在以往的研究中[23],有的學(xué)者嘗試?yán)眠x擇性激光熔融技術(shù)(Selective Laser Melting, SLM)制備具有多孔網(wǎng)狀結(jié)構(gòu)的TC4鈦合金下頜骨,產(chǎn)品的孔互聯(lián)良好,結(jié)構(gòu)無斷裂裂紋缺陷,孔隙率可控,產(chǎn)品質(zhì)量良好;當(dāng)將打印模型設(shè)計(jì)成鉆石分子的結(jié)構(gòu),小孔結(jié)構(gòu)0.2 mm,產(chǎn)品的結(jié)構(gòu)雖然存在一定的制造誤差,輪廓依然清晰;成品表面由于覆蓋球狀半熔融金屬顆粒,表面形貌凹凸不平。這些表面問題對(duì)3D打印技術(shù)來說似乎是難以避免,粗糙的表面造成了產(chǎn)品誤差。3D打印的TC4鈦合金產(chǎn)品,如果不經(jīng)過任何處理,直接用于部件之間的裝配,可能由于幾何尺寸和表面光潔度偏差大,造成裝配效果糟糕。解決的問題是在后處理中進(jìn)行拋光,然而拋光增加了成本,拋光過程難以控制拋光的厚度,使產(chǎn)品的尺寸出現(xiàn)不可控的精確度問題。產(chǎn)品出現(xiàn)的表面粗糙問題在部分醫(yī)用植入領(lǐng)域被認(rèn)為可以接受。在馮等人[24]的研究中顯示,3D打印的粗糙表面的種植體,更利于組織細(xì)胞的附著。雖然在其他方面粗糙表面可能會(huì)被限制使用。在臨床的實(shí)際使用中,見有報(bào)道利用3D打印成型的骨盆關(guān)節(jié)、骶骨、顱骨、足踝、關(guān)節(jié)假體、畸形矯正器和胸骨等,3D打印
均能快速成型,并植入人體需要的部位,與自然骨配合很好,均達(dá)到較理想的治療效果[25] [26] [27] [28][29]。雖然以上3D打印產(chǎn)品尺寸的具體偏差并未表明,但是證據(jù)已能夠表明3D打印的鈦合金產(chǎn)品質(zhì)量已能滿足部分臨床使用的要求。
3.2. 機(jī)械性能
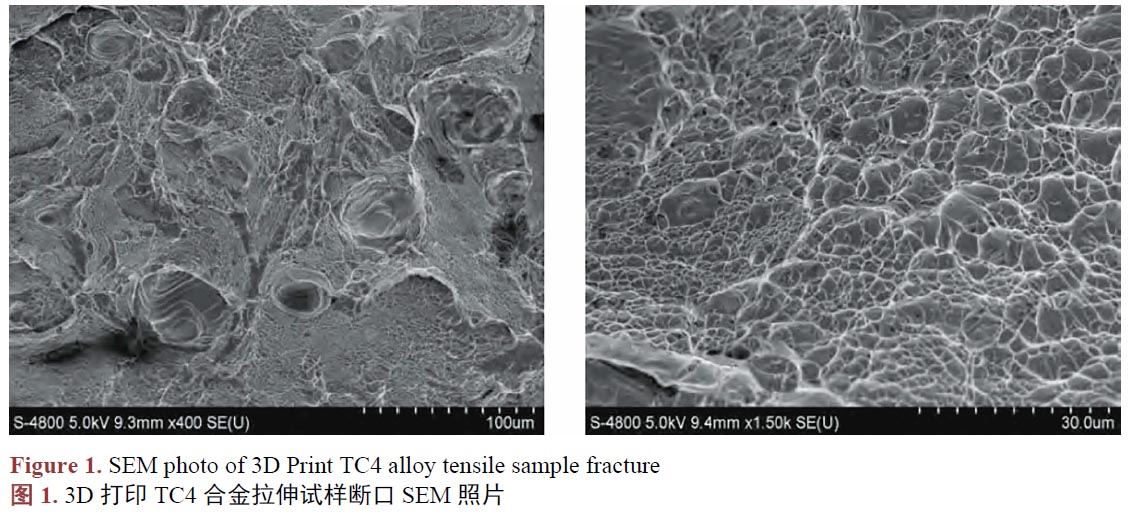
用于骨骼修復(fù)的TC4鈦合金人工骨,通常被運(yùn)用在必須承重的骨骼部位,需要能承受一定的載荷,所以要求TC4鈦合金人工骨必須具有足夠的機(jī)械性能。已有的研究[30]發(fā)現(xiàn),3D打印的TC4鈦合金件的機(jī)械性能并非都是一致的,不一樣的工藝參數(shù),會(huì)使產(chǎn)品的機(jī)械性能產(chǎn)生差異,良好的機(jī)械性能取決于合適的工藝參數(shù)[31]。張等人[32]研究3D打印的TC4鈦合金口腔修復(fù)產(chǎn)品的機(jī)械性能,結(jié)果發(fā)現(xiàn)3D打印完成的試件,未經(jīng)過任何處理,它們的維氏硬度值在372.93~428.46 HV 的范圍,高于傳統(tǒng)制造方式生產(chǎn)的TC4鈦合金的維氏硬度值[33]。抗拉強(qiáng)度、屈服強(qiáng)度和延伸率分別為1821.7 ± 146.2 MPa、1355.9 ± 109.7 MPa和31.3 ± 1.8%,機(jī)械性能保持著良好的狀態(tài),能滿足相關(guān)的國(guó)家標(biāo)準(zhǔn)[34]。3D打印金屬材料的過程不同于傳統(tǒng)的制造方法,它常使原材料經(jīng)歷著加熱–融化–冷卻的過程,并不斷在金屬分層重復(fù)這一過程,熔融后的金屬粉末,由于冷卻速率快速,會(huì)導(dǎo)致力學(xué)性能和幾何尺寸缺陷[35]。對(duì)于竣工后的產(chǎn)品進(jìn)行熱處理,被認(rèn)為是提高3D打印后的TC4鈦合金力學(xué)性能和幾何質(zhì)量的有效方法[36]。吳等人[37]對(duì)3D打印后進(jìn)行熱處理的TC4鈦合金件進(jìn)行拉伸強(qiáng)度測(cè)試,結(jié)果顯示它們的抗拉強(qiáng)度、屈服強(qiáng)度、延伸率等機(jī)械性能均優(yōu)于傳統(tǒng)制造方式的同類產(chǎn)品(表1),它們的耐磨損性能甚至高于天然牙齒的牙釉質(zhì)(表2),對(duì)其進(jìn)行拉伸斷裂的斷口進(jìn)行掃描電鏡(SEM)表征發(fā)現(xiàn),它們斷裂形式屬于韌性斷裂(圖1),證明其塑性良好。其他的報(bào)道中[38],研究人員稱利用SLM 技術(shù)制備的接骨板經(jīng)過簡(jiǎn)單的熱處理后其硬度高于鈦合金鑄件,極限拉伸強(qiáng)度、屈服強(qiáng)度和伸長(zhǎng)率均滿足常規(guī)鈦合金的力學(xué)要求。在朱、張等人[39] [40] [41] [42]的研究中,發(fā)現(xiàn)3D打印制造的TC4鈦合金產(chǎn)品,經(jīng)過一系列的后處理,在密度、強(qiáng)度、塑性、沖擊韌性和疲勞強(qiáng)度等指標(biāo)均可以與傳統(tǒng)方法制造的鈦合金件媲美甚至更優(yōu)。另一項(xiàng)研究中[43],利用3D打印制備孔徑約為400 μm 的TC4鈦合金支架,其極限抗壓強(qiáng)度和剪切強(qiáng)度均較致密結(jié)構(gòu)的TC4鈦合金材料有所下降,但接近人骨的力學(xué)性能,滿足人骨植入物的力學(xué)性能要求。在臨床使用的案例中[44],有多例患者使用了3D打印TC4鈦合金重建脊柱,治療因疾病造成的骨缺損,治療效果很好,在隨訪期間植入物并未發(fā)生斷裂等機(jī)械失效。
由此可見3D打印TC4鈦合金產(chǎn)品可以滿足人工骨材料的要求。
3.3. 生物相容性
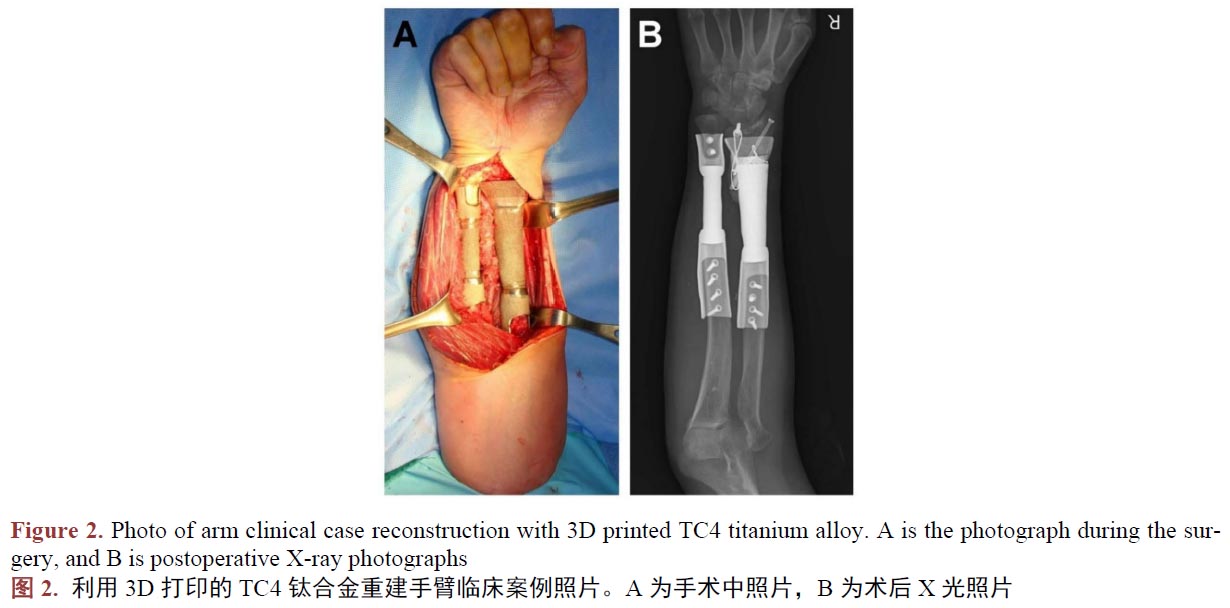
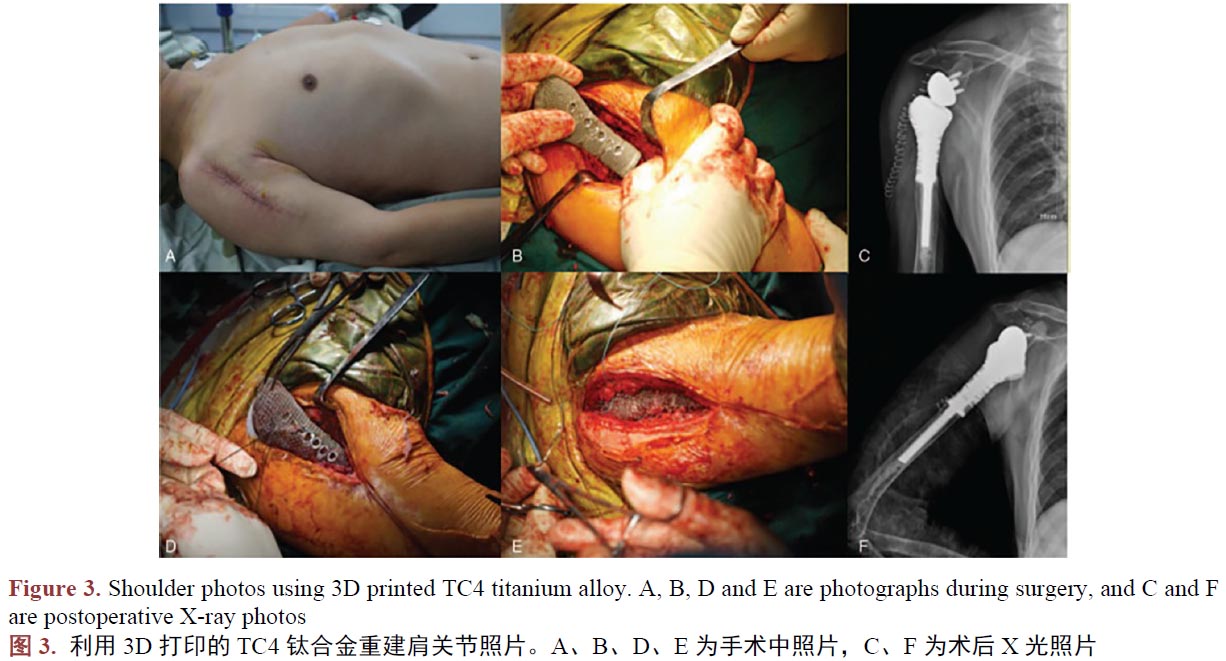
醫(yī)用鈦合金作為人體組織的替代物植入人體,與人體組織形成結(jié)合,須在人體內(nèi)不產(chǎn)生排異反應(yīng),所以新型工藝制備的鈦合金生物相容是評(píng)價(jià)其能否替代傳統(tǒng)工藝的另一個(gè)重要的指標(biāo)。在植入動(dòng)物體進(jìn)行3D打印TC4鈦合金件的生物相容性評(píng)估時(shí),學(xué)者們嘗試過將3D打印制備的蜂窩狀多孔TC4鈦合金支架、TC4牙種植體、骨干,植入兔、犬等動(dòng)物體,結(jié)果都指明了其在動(dòng)物體中的骨結(jié)合很穩(wěn)固,并未出現(xiàn)植入物周圍組織粘連、充血、水腫、壞死等令人擔(dān)憂的現(xiàn)象[45]-[50]。作為進(jìn)一步驗(yàn)證,臨床使用的案例研究中,學(xué)者們記錄和研究了臨床一些真實(shí)病人使用3D打印技術(shù)制備的TC4鈦合金產(chǎn)品,植入手臂(圖2) [51]、肩關(guān)節(jié)(圖3) [52]、頸椎骨[53]、脛骨[54] [55]、下頜骨等[56]部位,在研究期間發(fā)現(xiàn)3D打印技術(shù)制備TC4鈦合金人工骨具有較強(qiáng)的組織結(jié)合能力,且軟組織能牢固的附著于材料表面,其中所有患者沒有出現(xiàn)因假體的植入造成感染和排異反應(yīng)。所以我們認(rèn)為3D打印制備的鈦合金人工骨具有良好的生物相容性。
3.4. 生物安全性
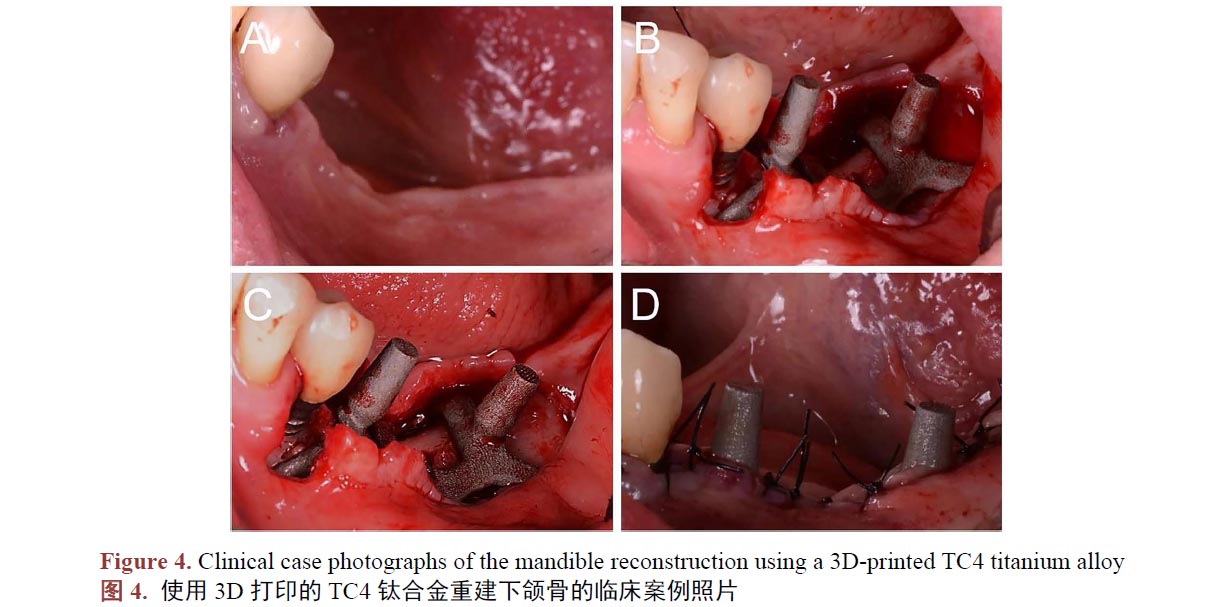
3D打印制備作為新型工藝,制備的人工骨材料,是否會(huì)對(duì)細(xì)胞具有毒性,或者是否會(huì)增加植入物感染等問題,也已被學(xué)者們進(jìn)行驗(yàn)證。已有報(bào)道稱在景、王、李等人[57] [58] [59] [60]的研究中,3D打印制備的TC4鈦合金對(duì)體外成骨細(xì)胞的體外增殖和分化沒有不良影響,沒有細(xì)胞毒性。王、肖等學(xué)者[61] [62] [63]研究得出3D打印制備的鈦合金種植體,對(duì)動(dòng)物體不具有亞慢性全身毒性,對(duì)動(dòng)物組織細(xì)胞無毒副作用,在動(dòng)物體內(nèi)不會(huì)發(fā)生明顯的材料磨損脫落。在臨床上,學(xué)者們發(fā)現(xiàn)3D打印制備的TC4鈦合金種植體生物安全性能良好,在多例病例中,病人下頜骨(圖4,A 為術(shù)前照片,B、C 為術(shù)中照片,D 為術(shù)后照片) [64] [65]、胸骨[66]、顱骨[67]、股骨[68]、脊椎[69]等重要部位植入3D打印的鈦合金產(chǎn)品,重建缺損部位,結(jié)果,大部分病人術(shù)后傷口愈合良好,研究的病例中僅有小部分病人發(fā)現(xiàn)植入物周圍感染,感染病人比例在正常范圍內(nèi),并未發(fā)現(xiàn)病例因?yàn)橹踩氩牧媳旧硪鸬母腥尽_@些證據(jù)表明3D打印的鈦合金件生物安全性是可靠的。
3.5. 耐腐蝕性能
鈦及其合金因其良好的耐腐蝕性能而聞名。然而鈦的耐腐蝕,并非該金屬具有高度不活潑的性質(zhì),實(shí)際上,鈦是一種較活潑的金屬元素,使鈦合金耐腐蝕的是它表面的二氧化鈦鈍化膜。氧化的二氧化鈦覆蓋于鈦合金表面形成致密的薄膜,使鈦合金鈍化從而阻止金屬進(jìn)一步腐蝕,達(dá)到抗腐蝕的功能[70] [71][72]。耐腐蝕性能的保持對(duì)種植體的機(jī)械性能和生物相容性的影響會(huì)種植體的整個(gè)使用壽命,過快的腐蝕會(huì)導(dǎo)致植入物過早的失效。作為一種先進(jìn)的智能制造技術(shù),3D打印的TC4鈦合金的耐腐蝕性能是否與傳統(tǒng)工藝制備的TC4鈦合金件一樣,是否能滿足臨床對(duì)此類金屬材料的腐蝕行為的要求一樣至關(guān)重要。
由于制造工藝的不同,馬等學(xué)者[73]發(fā)現(xiàn),3D打印制備的TC4鈦合金件的耐腐蝕性能明顯差于鍛造等傳統(tǒng)工藝制備的同種鈦合金。造成腐蝕性差的原因可能是,由于3D打印的鈦合金產(chǎn)品表面較粗糙導(dǎo)致,經(jīng)過進(jìn)一步的表面處理可以提高耐腐蝕性,解決這一問題,在J Fojt、白等學(xué)者[74] [75]的研究中已證實(shí)了這一觀點(diǎn)。然而對(duì)于人工骨材料,粗糙的表面可能更適用,拋光處理后耐腐蝕性能增強(qiáng),帶來的是生物活性變差。由此可見,3D打印的TC4鈦合金如果不經(jīng)過后續(xù)的表面修改處理,其耐腐蝕性能會(huì)明顯變差,但是可能帶來的是更好的生物活性,兩者應(yīng)該權(quán)衡取舍。上述的臨床案例并未發(fā)現(xiàn)產(chǎn)品在壽命內(nèi)由于腐蝕嚴(yán)重導(dǎo)致的失效。由此可見3D打印的TC4鈦合金的耐腐蝕性并非難以接受,或者可以選擇表面修飾進(jìn)一步補(bǔ)償耐腐蝕性能的不足。
4、3D打印鈦合金的表面改性
與傳統(tǒng)的加工工藝類似,3D打印技術(shù)只是改變了材料的加工的方式,并沒有從根本上改變材料的性質(zhì)。
所以 3D打印鈦合金產(chǎn)品中,鈦合金材料本身缺點(diǎn)依然存在.仍需要進(jìn)行進(jìn)一步的表面修飾。借鑒對(duì)常規(guī)加工的鈦合金表面改性的策略,可以選擇對(duì)3D打印鈦合金進(jìn)行機(jī)械改性、化學(xué)改性和復(fù)合材料[76] [77] [78]。
對(duì)用植入鈦合金,常用的機(jī)械改性的方法如表面噴砂、激光雕刻紋理等。噴砂和激光雕刻的目的是在鈦合金件表面制造凹凸地形。已有的研究顯示[79],凹凸地形的鈦合金表面具有一定的生物活性,能帶來更好的骨結(jié)合,并且不會(huì)對(duì)材料的力學(xué)性能和生物安全性帶來很大的影響,屬于較經(jīng)濟(jì)實(shí)用的表面改性方法。
常用的化學(xué)改性的方法有酸蝕、堿蝕、堿熱處理、表面氧化等,這樣的方式也是為了在TC4鈦合金表面發(fā)生化學(xué)反應(yīng),生成生物活性更好、耐腐蝕性能更強(qiáng)的物質(zhì)[80]。酸蝕、堿蝕能進(jìn)一步制造不規(guī)則的分層微、納米的多孔結(jié)構(gòu),并且在改性鈦表面生成生物活性更強(qiáng)耐腐蝕性能更好的TiO2,達(dá)到增強(qiáng)其表面的生物活性的同時(shí)提高材料的耐腐蝕性能[81] [82]。酸蝕和堿蝕的方法常常在噴砂后進(jìn)行,這樣做的目的既能清理鈦合金表面殘留的砂粒,又能將噴砂造成的粗糙表面進(jìn)一步活化。復(fù)合材料的方法是在鈦合金件表面復(fù)合一
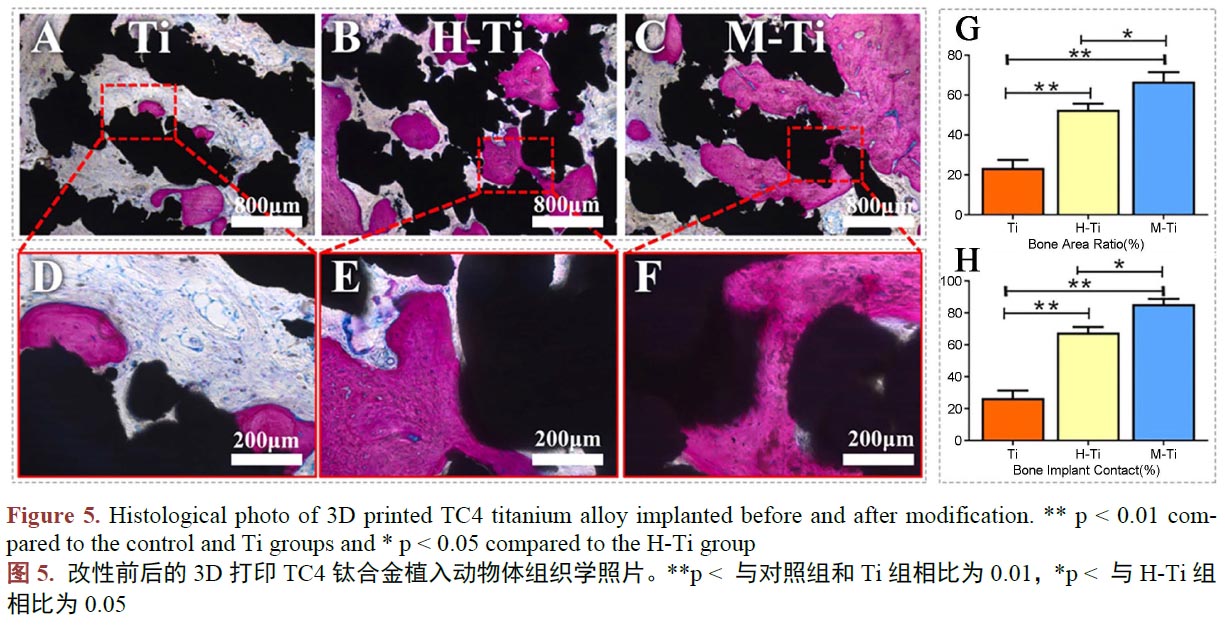
層具有特殊功能的材料,能達(dá)到改善鈦合金的耐腐蝕性、耐磨性、生物活性、抗菌性能等(圖5,A 和D、B 和E、C 和F 分別對(duì)應(yīng)的是改性前的鈦合金、利用水熱法在鈦合金表面制備Ca、P、O 涂層的改性鈦合金、利用微弧氧化在鈦合金表面制備Ca、P、O 涂層的改性鈦合金。G 為骨向種植體內(nèi)生長(zhǎng)的定量分析,H為骨與種植體接觸的定量分析。更多的骨向內(nèi)生長(zhǎng)和更多的骨與種植體接觸面積,證明對(duì)應(yīng)材料的生物活性更好。) [83] [84] [85]。復(fù)合材料常以涂層的方式涂覆于鈦合金基體表面,它常常使改性鈦合金兼具鈦合金本身優(yōu)異的性能和表面復(fù)合的材料的性能。常被用于復(fù)合在鈦合金基體表面,用于提高改性鈦的生物活性的材料有磷酸鈣、聚多巴胺等生物活性更好的材料。磷酸鈣優(yōu)秀的骨誘導(dǎo)性能已得到廣泛的認(rèn)可。在已有的研究中[86] [87] [88] [89],通過在鈦合金表面復(fù)合磷酸鈣、聚多巴胺等生物活性更好的材料,確實(shí)在一定程度上改善了改性鈦合金的生物活性。為更大限度的賦予涂層神奇的功能,可以在涂層中摻入一些金屬元素,使涂層具有抗菌、更強(qiáng)的生物活性的功效。其中,銀、鍶、鋅常被作為涂層的摻雜成分。TC4鈦合金種植體常由于不具備抗細(xì)菌感染的能力,這種功能的缺失會(huì)容易在進(jìn)行種植手術(shù)時(shí)發(fā)生細(xì)菌感染,造成植入手術(shù)的失敗,于是具有抗菌性能的復(fù)合涂層展現(xiàn)出了巨大的潛力。銀是一種天然的抗菌元素,有報(bào)道稱[90] [91] [92],在他們的研究中,在TC4鈦合金表面復(fù)合摻雜銀和鍶的羥基磷灰石涂層,使改性TC4鈦合金具有優(yōu)異的抗菌性能,同時(shí)擁有良好的生物活性。眾多的表面改性的效果表明,3D打印的TC4鈦合金人工骨,通過表面改性的方法,克服產(chǎn)品的缺點(diǎn),具有巨大的前途。
5、總結(jié)和展望
3D打印技術(shù)因特有的優(yōu)勢(shì),在非批量生產(chǎn)方面較傳統(tǒng)的制造工藝成本低,且能針對(duì)個(gè)性化進(jìn)行精準(zhǔn)加工,符合醫(yī)療學(xué)植入物的迫切需求。作為新型的加工工藝,3D打印的產(chǎn)品還不能完全被人們認(rèn)知和接受。上述大量的學(xué)者研究足以證明,3D打印的TC4鈦合金在產(chǎn)品質(zhì)量、機(jī)械性能、生物相容性、生物安全性和耐腐蝕性等方面的性能已能滿足部分使用要求。產(chǎn)品在一些臨床植入病人的案例中也顯示出了可喜的療效。說明3D打印TC4鈦合金人工骨完全可行。至今,3D打印的TC4鈦合金產(chǎn)品還存在一些尚未克服的問題,如產(chǎn)品精度仍然很有限、產(chǎn)品表面不光潔等。如果能完全解決上述的技術(shù)問題,3D打印將顛覆以往常規(guī)的加工方法,并刷新對(duì)TC4鈦合金人工骨的認(rèn)識(shí)。所以今后3D打印研究的重點(diǎn)可以放在提高加工精度和表面光潔度上。隨著3D打印技術(shù)和表面改性技術(shù)的進(jìn)步,優(yōu)化性能之后的TC4鈦合金人工骨將重新被人們考慮。3D打印全面取代傳統(tǒng)的制造工藝,進(jìn)行TC4鈦合金人工骨的制備,并非危言聳聽!
利益沖突聲明
作者聲明本文無利益沖突。
基金項(xiàng)目
本文由成都市醫(yī)學(xué)科研課題(2021043),四川省教育廳高等教育人才培養(yǎng)質(zhì)量和教學(xué)改革項(xiàng)目(JG2021-1102),教育部產(chǎn)學(xué)合作協(xié)同育人項(xiàng)目(202101011010),成都大學(xué)CC 國(guó)家眾創(chuàng)空間2021 年度創(chuàng)新創(chuàng)業(yè)教育專項(xiàng)課題(ccyg202101008), 四川省大學(xué)生創(chuàng)新創(chuàng)業(yè)訓(xùn)練計(jì)劃項(xiàng)目(S202111079028 ,S202111079043X,S202111079095,S202111079124X,S202111079041),成都大學(xué)大學(xué)生創(chuàng)新創(chuàng)業(yè)訓(xùn)練計(jì)劃項(xiàng)目(CDUCX2022604,CDUCX2022600)資助。
參考文獻(xiàn)
[1] 陳文杰. 3D打印工藝參數(shù)管理系統(tǒng)關(guān)鍵技術(shù)研究[D]: [碩士學(xué)位論文]. 徐州: 中國(guó)礦業(yè)大學(xué), 2019.
[2] Browne, M.P., Redondo, E. and Pumera, M. (2020) 3D Printing for Electrochemical Energy Applications. Chemical Reviews, 120, 2783-2810. https://doi.org/10.1021/acs.chemrev.9b00783
[3] Jamróz, W., Szafraniec, J., Kurek, M., et al. (2018) 3D Printing in Pharmaceutical and Medical Applications—RecentAchievements and Challenges. Pharmaceutical Research, 35, 1-22. https://doi.org/10.1021/acs.chemrev.9b00783
[4] Durfee, W.K. and Iaizzo, P.A. (2019) Medical Applications of 3D Printing. In: Iaizzo, P.A., Ed., Engineering in Medicine:Advances and Challenges, Academic Press, Cambridge, 527-543.
https://doi.org/10.1016/B978-0-12-813068-1.00021-X
[5] 施建平. 面向骨植入體3D打印的多孔結(jié)構(gòu)構(gòu)建研究[D]: [博士學(xué)位論文]. 南京: 東南大學(xué), 2018.
[6] 胡婧, 陶梅平, 唐金穎. 3D打印TC4鈦合金的成形工藝與熱處理行為研究[J]. 熱加工工藝, 2017, 46(16):220-224.
[7] 李豪杰. 3D打印與傳統(tǒng)加工TC4鈦合金組織與力學(xué)性能對(duì)比研究[D]: [碩士學(xué)位論文]. 北京: 北方工業(yè)大學(xué),2019.
[8] 吳棟. 關(guān)于TC4 基表面ZnO/HA 復(fù)合涂層的制備與性能研究[D]: [碩士學(xué)位論文]. 蘭州: 蘭州理工大學(xué), 2020.
[9] Ganesh, N. and Rambabu, S. (2021) Finite Element Analysis of Porous Ti-6Al-4V Alloy Structures for BiomedicalApplications. Journal of Physics: Conference Series, 2070, Article ID: 012224.
https://doi.org/10.1088/1742-6596/2070/1/012224
[10] Shen, X. and Shukla, P. (2020) A Review of Titanium Based Orthopaedic Implants (Part-I): Physical Characteristics,Problems and the Need for Surface Modification. International Journal of Peening Science and Technology, 1, 301-332.
[11] Zheng, J., Chen, L., Chen, D., et al. (2019) Effects of Pore Size and Porosity of Surface-Modified Porous TitaniumImplants on Bone Tissue Ingrowth. Transactions of Nonferrous Metals Society of China, 29, 2534-2545.
https://doi.org/10.1016/S1003-6326(19)65161-7
[12] Kapat, K., Maity, P.P., Rameshbabu, A.P., et al. (2018) Simultaneous Hydrothermal Bioactivation with Nano-TopographicModulation of Porous Titanium Alloys towards Enhanced Osteogenic and Antimicrobial Responses. Journal of MaterialsChemistry B, 6, 2877-2893.
https://doi.org/10.1039/C8TB00382C
[13] McGilvray, K.C., Easley, J., Seim, H.B., et al. (2018) Bony Ingrowth Potential of 3D-Printed Porous Titanium Alloy:A Direct Comparison of Interbody Cage Materials in an in Vivo Ovine Lumbar Fusion Model. The Spine Journal, 18,1250-1260.
https://doi.org/10.1016/j.spinee.2018.02.018
[14] Xia, Y., Feng, C., Xiong, Y., et al. (2019) Mechanical Properties of Porous Titanium Alloy Scaffold Fabricated UsingAdditive Manufacturing Technology. International Journal of Applied Electromagnetics and Mechanics, 59, 1087-1095.
https://doi.org/10.3233/JAE-171197
[15] 趙立明. 3D打印鈦合金骨小梁骨干假體在山羊體內(nèi)骨長(zhǎng)入的實(shí)驗(yàn)研究[D]: [碩士學(xué)位論文]. 天津: 天津醫(yī)科大學(xué), 2017.
[16] 張?zhí)m, 王翔, 劉軍, 等. 3D打印鈦合金骨小梁多孔結(jié)構(gòu)的拉伸性能[J]. 中國(guó)組織工程研究, 2020, 24(22):3498-3503.
[17] Hedia, H.S., Aldousari, S.M., Timraz, H.A., et al. (2019) Stress Shielding Reduction via Graded Porosity of a Femoral Stem Implant. Materials Testing, 61, 695-704. https://doi.org/10.3139/120.111374
[18] Al-Tamimi, A.A. (2021) 3D Topology Optimization and Mesh Dependency for Redesigning Locking Compression Plates Aiming to Reduce Stress Shielding. International Journal of Bioprinting, 7, 339-348.
https://doi.org/10.18063/ijb.v7i3.339
[19] 李崇崇, 付步芳, 杜曉丹, 等. 3D打印個(gè)體化骨盆假體多孔結(jié)構(gòu)物理性能檢測(cè)方法研究[J]. 生物醫(yī)學(xué)工程與臨床, 2020, 24(2): 126-130.
[20] Zheng, Y., Han, Q., Wang, J., et al. (2020) Promotion of Osseointegration between Implant and Bone Interface by Titanium Alloy Porous Scaffolds Prepared by 3d Printing. ACS Biomaterials Science & Engineering, 6, 5181-5190.
https://doi.org/10.1021/acsbiomaterials.0c00662
[21] 孫星. 3D打印可再生多孔骨骼支架及性能研究[D]: [碩士學(xué)位論文]. 濟(jì)南: 山東建筑大學(xué), 2020.[22] Wallace, N., Schaffer, N.E., Aleem, I.S., et al. (2020) 3D-Printed Patient-Specific Spine Implants: A Systematic Review.Clinical Spine Surgery, 33, 400-407.
https://doi.org/10.1097/BSD.0000000000001026
[23] 彭文明, 劉云峰, 包霆威, 等. 3D打印多孔鈦合金骨植入體設(shè)計(jì)制造研究[C]//中華口腔醫(yī)學(xué)會(huì). 第十六次全國(guó)口腔醫(yī)學(xué)數(shù)字化學(xué)術(shù)會(huì)議暨中華口腔醫(yī)學(xué)會(huì)第四屆口腔醫(yī)學(xué)計(jì)算機(jī)專業(yè)委員會(huì)第二次全體委員會(huì)議論文匯編.2018: 19-20.
[24] 馮辰棟, 夏宇, 李祥, 等. 3D打印多孔鈦支架微觀孔隙結(jié)構(gòu)和力學(xué)性能[J]. 醫(yī)用生物力學(xué), 2017, 32(3): 256-260.
[25] Zhao, X., Xiao, J., Sun, Y., et al. (2018) Novel 3D Printed Modular Hemipelvic Prosthesis for Successful HemipelvicArthroplasty: A Case Study. Journal of Bionic Engineering, 15, 1067-1074.
https://doi.org/10.1007/s42235-018-0094-9
[26] Wei, R., Guo, W., Ji, T., et al. (2017) One-Step Reconstruction with a 3D-Printed, Custom-Made Prosthesis after Total En Bloc Sacrectomy: A Technical Note. European Spine Journal, 26, 1902-1909.
https://doi.org/10.1007/s00586-016-4871-z
[27] Ameen, W., Al-Ahmari, A., Mohammed, M.K., et al. (2018) Design, Finite Element Analysis (FEA), and Fabrication
of Custom Titanium Alloy Cranial Implant Using Electron Beam Melting Additive Manufacturing. Advances in Production
Engineering & Management, 13, 267-278. https://doi.org/10.14743/apem2018.3.289
[28] Dekker, T.J., Steele, J.R., Federer, A.E., et al. (2018) Use of Patient-Specific 3D-Printed Titanium Implants for Complex
Foot and Ankle Limb Salvage, Deformity Correction, and Arthrodesis Procedures. Foot & Ankle International,
39, 916-921. https://doi.org/10.1177/1071100718770133
[29] Wu, Y., Chen, N., Xu, Z., et al. (2018) Application of 3D Printing Technology to Thoracic Wall Tumor Resection and
Thoracic Wall Reconstruction. Journal of Thoracic Disease, 10, 6880-6890. https://doi.org/10.21037/jtd.2018.11.109
[30] Yi, T., Zhou, C., Ma, L., et al. (2020) Direct 3-D Printing of Ti-6Al-4V/HA Composite Porous Scaffolds for Customized
Mechanical Properties and Biological Functions. Journal of Tissue Engineering and Regenerative Medicine, 14,
486-496. https://doi.org/10.1002/term.3013
[31] Kushwaha, A., Kumar, S.A. and Velu, R. (2021) Selective Laser Melting of Titanium Alloys: Effect of Processing Parameters
on Microstructure and Mechanical Properties. International Journal of Mechatronics and Manufacturing Systems,
14, 128-142. https://doi.org/10.1504/IJMMS.2021.119156
[32] 張瑋航, 張虎, 李英姿, 等. 3D打印激光快速成型牙種植體的制備及其機(jī)械性能分析[J]. 吉林大學(xué)學(xué)報(bào)(醫(yī)學(xué)版),
2017, 43(1): 52-56+216.
[33] Jia, L.M., Liu, R.L., Liang, Z.M., et al. (2016) Research of Structure and Hardness of TC4 Alloy for Centrifugal Cast
ings. Advanced Material Engineering: Proceedings of the 2015 International Conference on Advanced Material Engineering,
Guangzhou, 15-17 May 2015, 301-307. https://doi.org/10.1142/9789814696029_0036
[34] 楊群, 陳長(zhǎng)勝, 馬忠賢, 王劍. GB/T 13810-2017《外科植入物用鈦及鈦合金加工材》標(biāo)準(zhǔn)解析[J]. 中國(guó)醫(yī)療器械
信息, 2019, 25(1): 14-15+53.
[35] 馬濤. 激光選區(qū)熔化成形Ti-6Al-4V 疲勞性能研究[D]: [碩士學(xué)位論文]. 南京: 南京理工大學(xué), 2019.
[36] Fousova, M. and Vojtech, D. (2018) Thermal Treatment of 3D-Printed Titanium Alloy. Manufacturing Technology,
18, 227-232. https://doi.org/10.21062/ujep/82.2018/a/1213-2489/MT/18/2/227
[37] 吳文孟, 張倩, 寧寶麟, 等. 3D打印Ti-6Al-4V 合金機(jī)械性能研究[J]. 全科口腔醫(yī)學(xué)電子雜志, 2016, 3(10): 93-95.
[38] Wang, D., Wang, Y., Wu, S., et al. (2017) Customized a Ti6Al4V Bone Plate for Complex Pelvic Fracture by Selective
Laser Melting. Materials, 10, 35-48. https://doi.org/10.3390/ma10010035
[39] 朱加雷, 王凱, 馬桂殿, 等. TC4鈦合金激光選區(qū)熔化成形性能研究[J]. 應(yīng)用激光, 2017, 37(6): 793-800.
[40] Skvortsova, S.V., German, M.A. and Spektor, V.S. (2019) Structure and Properties of Alloy Ti-6Al-4V Samples Fabricated
by 3D Printing. Russian Metallurgy (Metally), 2019, 863-872. https://doi.org/10.1134/S0036029519090106
[41] Smith, K.E., Dupont, K.M., Safranski, D.L., et al. (2016) Use of 3D Printed Bone Plate in Novel Technique to Surgically
Correct Hallux Valgus Deformities. Techniques in Orthopaedics (Rockville, Md.), 31, 181-189.
https://doi.org/10.1097/BTO.0000000000000189
[42] Zhang, C., Zhang, L., Liu, L., et al. (2020) Mechanical Behavior of a Titanium Alloy Scaffold Mimicking Trabecular
Structure. Journal of Orthopaedic Surgery and Research, 15, 1-11. https://doi.org/10.1186/s13018-018-1031-7
[43] 劉暢, 王辰宇, 劉賀, 等. 3D打印Ti6Al4V 鈦合金支架的力學(xué)性能及生物相容性[J]. 中國(guó)有色金屬學(xué)報(bào), 2018,
28(4): 758-765.
[44] Girolami, M., Boriani, S., Bandiera, S., et al. (2018) Biomimetic 3D-Printed Custom-Made Prosthesis for Anterior
Column Reconstruction in the Thoracolumbar Spine: A Tailored Option Following En Bloc Resection for Spinal Tumors.
European Spine Journal, 27, 3073-3083. https://doi.org/10.1007/s00586-018-5708-8
[45] 芮敏, 鄭欣, 張?jiān)茟c, 等. 3D打印多孔鈦合金支架修復(fù)兔橈骨骨缺損[J]. 中國(guó)組織工程研究, 2019, 23(18):
2789-2793.
[46] Tu, C.C., Tsai, P.I., Chen, S.Y., et al. (2020) 3D Laser-Printed Porous Ti6Al4V Dental Implants for Compromised
Bone Support. Journal of the Formosan Medical Association, 119, 420-429. https://doi.org/10.1016/j.jfma.2019.07.023
[47] 王蕊, 李美華, 周萬琳. 3D打印鈦合金種植體的制備及其骨結(jié)合性能[J]. 吉林大學(xué)學(xué)報(bào)(醫(yī)學(xué)版), 2021, 47(1):
82-88.
[48] 周萬琳. 選擇性激光燒結(jié)3D打印鈦合金種植體的制備及其體內(nèi)研究[D]: [碩士學(xué)位論文]. 長(zhǎng)春: 吉林大學(xué),
2019.
[49] 張劍鋒. 3D打印組配式節(jié)段型人工假體重建骨干缺損的實(shí)驗(yàn)研究[D]: [博士學(xué)位論文]. 天津: 天津醫(yī)科大學(xué),
2019.
[50] 向健, 楊立峰, 田勝慧, 等. 新型3D打印骨修復(fù)體的骨組織相容性研究[J]. 中國(guó)醫(yī)學(xué)工程, 2018, 26(10): 26-29.
[51] Park, J.W., Song, C.A., Kang, H.G., et al. (2020) Integration of a Three-Dimensional-Printed Titanium Implant in
Human Tissues: Case Study. Applied Sciences, 10, 553-561. https://doi.org/10.3390/app10020553
[52] Zou, Y., Yang, Y., Han, Q., et al. (2018) Novel Exploration of Customized 3D Printed Shoulder Prosthesis in Revision
of Total Shoulder Arthroplasty: A Case Report. Medicine, 97, e13282-e13288.
https://doi.org/10.1097/MD.0000000000013282
[53] Wei, F., Li, Z., Liu, Z., et al. (2020) Upper Cervical Spine Reconstruction Using Customized 3D-Printed Vertebral
Body in 9 Patients with Primary Tumors Involving C2. Annals of Translational Medicine, 8, 332-340.
https://doi.org/10.21037/atm.2020.03.32
[54] Guder, W.K., Hardes, J., Nottrott, M., et al. (2021) Highly Cancellous Titanium Alloy (TiAl6V4) Surfaces on
Three-Dimensionally Printed, Custom-Made Intercalary Tibia Prostheses: Promising Short- to Intermediate-Term Results.
Journal of Personalized Medicine, 11, 351-360. https://doi.org/10.3390/jpm11050351
[55] Zhang, Y., Zhang, L., Sun, R., et al. (2018) A new 3D Printed Titanium Metal Trabecular Bone Reconstruction System
for Early Osteonecrosis of the Femoral Head. Medicine, 97, e11088-e11096.
https://doi.org/10.1097/MD.0000000000011088
[56] Park, J.H., Odkhuu, M., Cho, S., et al. (2020) 3D-Printed Titanium Implant with Pre-Mounted Dental Implants for
Mandible Reconstruction: A Case Report. Maxillofacial Plastic and Reconstructive Surgery, 42, 1-4.
[57] 景麗, 史文, 曹雨, 等. 3D打印鈦合金多孔材料對(duì)體外成骨細(xì)胞系MC3T3-E1 的生物安全性[J]. 基礎(chǔ)醫(yī)學(xué)與臨
床, 2020, 40(10): 1374-1380.
[58] 王驊, 王鷂, 張彪. 3D打印鈦合金牙種植體的細(xì)胞毒性的研究[J]. 中國(guó)口腔種植學(xué)雜志, 2019, 24(1): 10-13.
[59] 李改明, 劉思雨, 戰(zhàn)德松, 等. 3D打印醫(yī)用鈦合金的抗菌性能和體外生物相容性[J]. 材料研究學(xué)報(bào), 2019, 33(2):
117-123.
[60] 李軍, 魏建華, 張玉梅, 等. 新型醫(yī)用鈦合金生物相容性評(píng)價(jià)[J]. 實(shí)用口腔醫(yī)學(xué)雜志, 2010, 26(5): 636-640.
[61] 王涵, 趙丹妹, 許建霞, 等. 3D打印骨科鈦合金的亞慢性全身毒性研究[J]. 組織工程與重建外科雜志, 2020,
16(1): 6-10.
[62] Chioibasu, D., Achim, A., Popescu, C., et al. (2019) Prototype Orthopedic Bone Plates 3D Printed by Laser Melting
Deposition. Materials, 12, 906-925. https://doi.org/10.3390/ma12060906
[63] 肖維維. 3D打印鈦合金下頜骨接骨板的有效性和安全性的初步研究[D]: [碩士學(xué)位論文]. 西安: 中國(guó)人民解放
軍空軍軍醫(yī)大學(xué)口腔醫(yī)學(xué)院, 2018.
[64] Mangano, C., Bianchi, A., Mangano, F.G., et al. (2020) Custom-Made 3D Printed Subperiosteal Titanium Implants for
the Prosthetic Restoration of the Atrophic Posterior Mandible of Elderly Patients: A Case Series. 3D Printing in Medicine,
6, 1-14. https://doi.org/10.1186/s41205-019-0055-x
[65] Popovski, V., Benedetti, A., Panchevski, G., et al. (2020) Emergency State of Mandible Fracture Management in
COVID-19 Pandemic Area: A Case Report. Journal of Morphological Sciences, 3, 107-113.
[66] Goldsmith, I., Evans, P.L., Goodrum, H., et al. (2020) Chest Wall Reconstruction with an Anatomically Designed 3-D
Printed Titanium Ribs and Hemi-Sternum Implant. 3D Printing in Medicine, 6, 26.
https://doi.org/10.1186/s41205-020-00079-0
[67] Park, E.K., Lim, J.Y., Yun, I.S., et al. (2016) Cranioplasty Enhanced by Three-Dimensional Printing: Custom-Made
Three-Dimensional-Printed Titanium Implants for Skull Defects. Journal of Craniofacial Surgery, 27, 943-949.
https://doi.org/10.1097/SCS.0000000000002656
[68] Zhang, Y., Zhang, L.L., Xiang, H., et al. (2017) The Effectiveness of 3D Printed Titanium Alloy Trabecular Reconstruction
rod for the Treatment of Early Osteonecrosis of Femoral Head. Tianjin Medical Journal, 45, 1222-1227.
[69] Zhou, H., Liu, S., Li, Z., et al. (2022) 3D-Printed Vertebral Body for Anterior Spinal Reconstruction in Patients with
Thoracolumbar Spinal Tumors. Journal of Neurosurgery: Spine, 1, 1-9. https://doi.org/10.3171/2022.1.SPINE21900
[70] Geetha, M., Singh, A.K., Asokamani, R., et al. (2009) Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—
A Review. Progress in Materials Science, 54, 397-425. https://doi.org/10.1016/j.pmatsci.2008.06.004
[71] Bocchetta, P., Chen, L.Y., Tardelli, J.D.C., et al. (2021) Passive Layers and Corrosion Resistance of Biomedical
Ti-6Al-4V and β-Ti Alloys. Coatings, 11, 487-518. https://doi.org/10.3390/coatings11050487
[72] Saini, M., Singh, Y., Arora, P., et al. (2015) Implant Biomaterials: A Comprehensive Review. World Journal of Clinical
Cases: WJCC, 3, 52-57. https://doi.org/10.12998/wjcc.v3.i1.52
[73] Mah, D., Pelletier, M.H., Lovric, V., et al. (2019) Corrosion of 3D-Printed Orthopaedic Implant Materials. Annals of
Biomedical Engineering, 47, 162-173. https://doi.org/10.1007/s10439-018-02111-1
[74] Fojt, J., Hybá?ek, V., Ka?enka, Z., et al. (2020) Influence of Surface Finishing on Corrosion Behaviour of 3D Printed
TiAlV Alloy. Metals, 10, 1547-1557. https://doi.org/10.3390/met10111547
[75] Bai, C., Li, P., Gang, T., et al. (2021) Influence of Processing Technology on Electrochemical Corrosion Behavior of
Ti-6Al-4V Alloys. Corrosion, 77, 402-412. https://doi.org/10.5006/3490
[76] Liang, C.Y., Jiang, X.J., Ji, R.L., et al. (2021) Preparation and Surface Modification of 3D Printed Ti-6Al-4V Porous
Implant. Rare Metals, 40, 1164-1172. https://doi.org/10.1007/s12598-020-01413-5
[77] Xu, J., Zhang, J., Shi, Y., et al. (2022) Surface Modification of Biomedical Ti and Ti Alloys: A Review on Current
Advances. Materials, 15, 1749-1777. https://doi.org/10.3390/ma15051749
[78] Wang, Q., Zhou, P., Liu, S., et al. (2020) Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration:
A Review. Nanomaterials, 10, 1244-1270. https://doi.org/10.3390/nano10061244
[79] Demirci, S., Dikici, T. and Güllüo?lu, A.N. (2022) Micro/Nanoscale Surface Modification of Ti6Al4V Alloy for Implant
Applications. Journal of Materials Engineering and Performance, 31, 1503-1511.
https://doi.org/10.1007/s11665-021-06232-y
[80] Cheung, K.H., Pabbruwe, M.B., Chen, W.F., et al. (2021) Thermodynamic and Microstructural Analyses of Photocatalytic
TiO2 from the Anodization of Biomedical-Grade Ti6Al4V in Phosphoric Acid or Sulfuric Acid. Ceramics International,
47, 1609-1624. https://doi.org/10.1016/j.ceramint.2020.08.277
[81] Cervino, G., Fiorillo, L., Iannello, G., et al. (2019) Sandblasted and Acid Etched Titanium Dental Implant Surfaces
https://doi.org/10.3390/ma12111763
[82] Luo, Y., Jiang, Y., Zhu, J., et al. (2020) Surface Treatment Functionalization of Sodium Hydroxide onto 3D Printed
Porous Ti6Al4V for Improved Biological Activities and Osteogenic Potencies. Journal of Materials Research and
Technology, 9, 13661-13670. https://doi.org/10.1016/j.jmrt.2020.09.076
[83] Afrouzian, A., Avila, J.D. and Bandyopadhyay, A. (2021) Biotribocorrosion of 3D-Printed Silica-Coated Ti6Al4V for
Load-Bearing Implants. Journal of Materials Research, 36, 3974-3984. https://doi.org/10.1557/s43578-021-00277-4
[84] Huang, L., Cai, B., Huang, Y., et al. (2021) Comparative Study on 3D Printed Ti6Al4V Scaffolds with Surface Modifications
Using Hydrothermal Treatment and Microarc Oxidation to Enhance Osteogenic Activity. ACS Omega, 6,
1465-1476. https://doi.org/10.1021/acsomega.0c05191
[85] Shanmugapriya, P., Srinivasan, V., Karthikeyan, B., et al. (2020) Wear Study on Sol-Gel-Coated Ti-6Al-4V Alloy.
Journal of Bio- and Tribo-Corrosion, 6, 1-12. https://doi.org/10.1007/s40735-020-00423-1
[86] Qin, J., Yang, D., Maher, S., et al. (2018) Micro- and Nano-Structured 3D Printed Titanium Implants with a Hydroxyapatite
Coating for Improved Osseointegration. Journal of Materials Chemistry B, 6, 3136-3144.
https://doi.org/10.1039/C7TB03251J
[87] Wang, C., Hu, H., Li, Z., et al. (2019) Enhanced Osseointegration of Titanium Alloy Implants with Laser Microgrooved
Surfaces and Graphene Oxide Coating. ACS applied materials & interfaces, 11, 39470-39483.
https://doi.org/10.1021/acsami.9b12733
[88] Li, L., Li, Y., Yang, L., et al. (2019) Polydopamine Coating Promotes Early Osteogenesis in 3D Printing Porous Ti6Al4V
Scaffolds. Annals of Translational Medicine, 7, 240-253. https://doi.org/10.21037/atm.2019.04.79
[89] Wang, S., Li, R., Li, D., et al. (2018) Fabrication of Bioactive 3D Printed Porous Titanium Implants with Sr
ion-Incorporated Zeolite Coatings for Bone Ingrowth. Journal of Materials Chemistry B, 6, 3254-3261.
https://doi.org/10.1039/C8TB00328A
[90] Fielding, G.A., Roy, M., Bandyopadhyay, A., et al. (2012) Antibacterial and Biological Characteristics of Silver Containing
and Strontium Doped Plasma Sprayed Hydroxyapatite Coatings. Acta Biomaterialia, 8, 3144-3152.
https://doi.org/10.1016/j.actbio.2012.04.004
[91] Wang, B., Ma, L., Xie, L., et al. (2020) Chemical Stability, Antibacterial and Osteogenic Activities Study of Strontium-
Silver Co-Substituted Fluorohydroxyapatite Nanopillars: A Potential Multifunctional Biological Coating. Ceramics
International, 46, 27758-27773. https://doi.org/10.1016/j.ceramint.2020.07.275
[92] O’Sullivan, C., O’Neill, L., O’Leary, N.D., et al. (2021) Osteointegration, Antimicrobial and Antibiofilm Activity of
Orthopaedic Titanium Surfaces Coated with Silver and Strontium-Doped Hydroxyapatite Using a Novel Blasting
Process. Drug Delivery and Translational Research, 11, 702-716. https://doi.org/10.1007/s13346-021-00946-1
相關(guān)鏈接